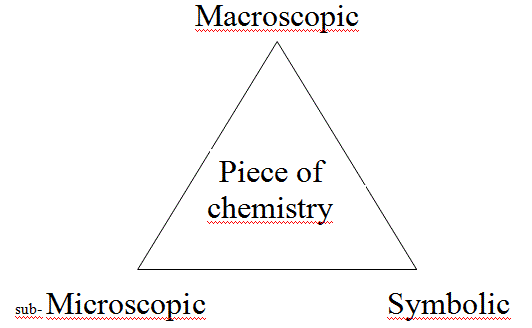

A piece of chemistry (properties of a chemical compound, a chemical reaction etc.) can be described on different levels. To think of three different levels (cf. those pictured above) can be useful.
"The top of the iceberg" - the macroscopic level - is what we can see and sense (also including the use of some instrument to extend the power of our senses) i.e. amount colour, smell, consistency, temperature, the number of phases (e.g. is there a precipitate?) and changes in these properties. The use of certain instruments makes it possible to quantify the qualitative observations. What we can do at all is to observe and measure; and it is what we should do carefully and thouroughly in the first place.
EX: If you add drops of a solution of silver nitrate to a solution of sodium chloride in dilute nitric acid you observe the formation of a white precipitate of silver chloride
Formula and correlations between entities are used to describe the chemical phenomena. This is the symbolic level .
EX(cont.): Ag+ + Cl- ® AgCl(s) ; pLAgCl=10; are two descriptions on the symbolic lecvel of the above phenomenum; first, silver chloride is insoluble in aqueous acid and neither the sodium ions nor the nitrate ions participate in the reaction. Secondly, the solubility can be quantified. To do so, you have to know, that "p" in pL is an operator meaning: take the log10 to the number and subtract the result from zero; and that L is the symbol for the entity "solubility product", the index of L specifying the formula of the compound in question.
The phenomena are "explained" or rationalised on the microscopic level. Concepts and models are used to communicate about our imaginations of the nature of structure of matter and of why, how, how fast and how effectively compounds react with each other.
EX(cont.): Silver ions and chloride ions are hydrated in aqueous solution. Especially the silver ions are not hydrated firmly. Silver ions and chloride ions form stronger bonds with a covalent contribution because of the polarisability of the electroncluod of the ions. The crystal lattice of silver chloride is reasonable stabile among other factors because it has the sodium chloride structure. The kinetics of precipitation is fast and the first step is more or less diffusion controlled partly becaouse the ions are spherical. The equilibrium is.... (and so on and so on)
When a chemical phenomenon is to be predicted (e.g. " what happens when 1 liter of concentrated nitric acid is mixed with 1 liter of concentrated ammonia") it is important to be aware of to which level of description the answer belongs, and make the prediction more complete by turning to another level of description of the same phenomena.