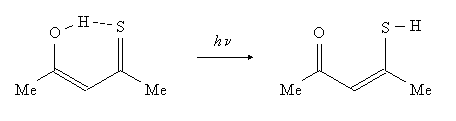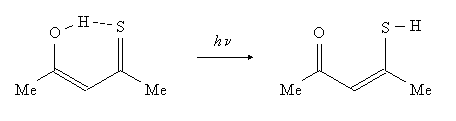Thioacetylacetone: Structural and vibrational assignments
ChemPhysChem 5, 495-502 (2004)
Y. Posokhov,a A. Gorski,a J. Spanget-Larsen,b F. Duus,b P.E. Hansen,b and J. Waluka
aInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44, 01-224 Warsaw, Poland
bDepartment of Life Sciences & Chemistry, Roskilde
University, P.O.Box 260, DK-4000 Roskilde, Denmark
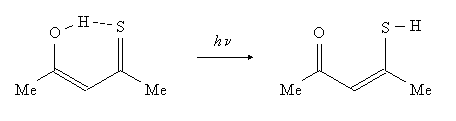
Abstract
Thioacetylacetone (TAA) and its variously deuteriated isotopomers have been investigated using electronic and vibrational spectroscopy combined with quantum chemical calculations. The compound is known for its photochromic properties, but the structure of its initial and final form has been the subject of a long debate. Analysis of the IR spectra recorded in low-temperature argon and xenon matrices, room temperature solutions and in the gas phase allowed us to establish the nature of the ground state species and its photoproduct. Similar to the case of another β-thioxoketone, thiodibenzoylmethane, the dominant ground state configuration corresponds to the chelated (Z)-enol tautomeric form, whereas the photoproduct can be assigned to the non-chelated SH exo-rotamer of the (Z)-enethiol tautomeric form. A small fraction of the SH endo-rotameric chelated (Z)-enethiol form has also been detected under unperturbed conditions. Relative to the stable (Z)-enol tautomer, the endo- and exo-(Z)-enethiol rotamers are predicted to be 1.30 and 3.88 kcal/mol more energetic (B3LYP/cc-pVTZ, ground state E + ZPE):
Detailed vibrational assignments have been proposed for the photoproduct and for its precursor, based on quantum chemical calculations and polarization experiments. In the case of the chelated (Z)-enol species prevailing in the ground state, a second order perturbative anharmonic analysis at the B3LYP/cc-pVTZ level indicated strong anharmonic effects associated with the intra molecular hydrogen bond, leading to a shift of more than 600 cm-1 of the wavenumber of the OH-stretching vibration.