Crystal structures, based on close-packing of spheres , octahedral and tetrahedras holes.
If we consider the cubic close-packed (CCP) arrangement we have a simple figure with 8 equal spheres in the corners of a cube and 6 equal spheres in the middle of the 6 faces of the cube.

In this figure, which is also called Face Centered Cubic there is one octahedral hole per sphere and two tetrahedral holes per sphere.
If other spheres are places in such holes we get representations of some crystal structures.
Blue spheres may be placed in every second tetrahedral hole:
|
|
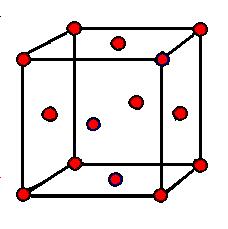
In this figure there is 1 red sphere in every corner, each of which belongs to 8 individual, but equal cubes. Further there are 1 red sphere on each ot the faces, each of which belongs to two induvidual, but equal cubes. 8/8+6/2=4 red spheres. In this figure there are exactly 4 blue spheres and it represent a way to arrange equal number of two different particles. We call it a 4:4 arrangement or a tetrahedral configuration. ZnS has this structure. |
|
|
This arrangement is related to the structure above, in that all the tetrahedral holes are occupied by blue spheres. We have a 4:8 stoichiometry: Each og the blue spheres are tetrahedrally surrounded by 4 red ones. Each red sphere is surrounded by 8 blue spheres in a cubic arrangement. CaF2 has this structure. |
|
|
Here a blue sphere occupies every octahedral hole. There are 6 nearest nieghbours. NaCl has this structure |
|
|
In this figure 2/3 of the octahedral holes in the network of blue spheres are occupied by red spheres. Al2O3 has this structure. Other crystals, where the two metal ions are different have this structure e.g. Ilmenite, FeTiO3. The condition is that the two different ions are not too different in size |
|
|
Here are 6/2 greenish blue spheres and 8/8 red spheres in the network and a black sphere in the middle. There are three differently coloured spheres in the FCC-structure. In Perowskite, CaTiO3, there is a large difference in size of the two cations, and the little Ti(IV) in the middle is surrounded octahedrally of greenish blue oxide anions, each of which in turn is surrounded by 4 red Ca2+ ions. The other way round, each red sphere is surrounded by 3x8/2 = 12 greenish blue spheres. |